-
Author
Michael Liu -
Co-author
Nele Vandersickel, Alexander V Panfilov, Zhilin Qu
-
Title
Arrhythmogenesis in Long QT Syndrome: Mechanistic and Therapeutic Insight from an in silico Human Model
-
Abstract
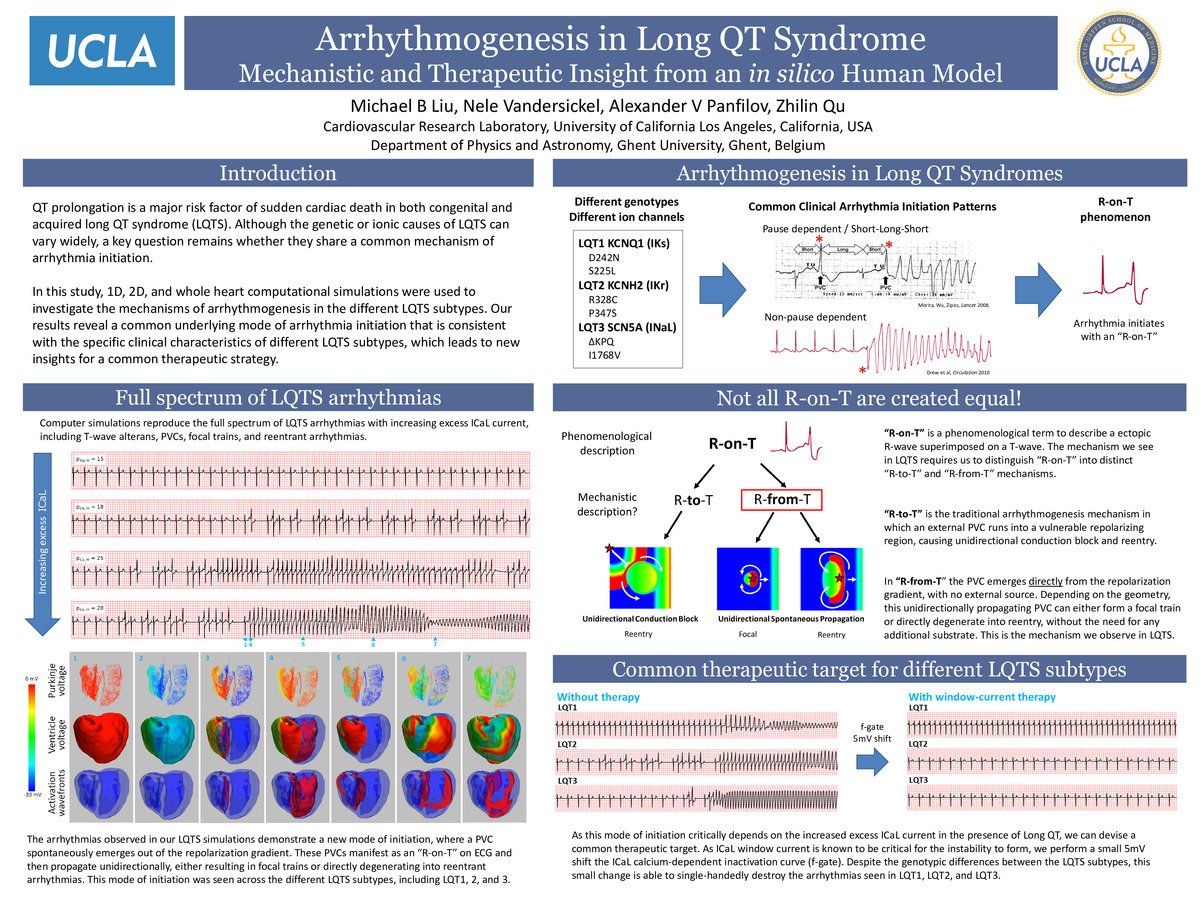
The genetic and ionic causes of long QT syndrome (LQTS) can vary widely in both congenital and acquired LQTS, but they all share a major risk factor of sudden cardiac death due to arrhythmias. Despite this, several differences in arrhythmogenesis have been noted between the specific LQTS subtypes in the clinical literature. For example, it has been noted that LQT2 arrhythmia onset is usually pause-dependent, while LQT1 arrhythmias are usually pause-independent and LQT3 is somewhat mixed (Tan et al, Circulation 2006). Additionally, the specific triggers of lethal arrhythmias in LQT2 and LQT3 are predominantly caused by sleep and emotional events, while in LQT1 they are primarily exercise related (Schwartz et al, Circulation 2001). This brings up a key question: Do the seemingly different clinical characteristics of each LQTS imply different arrhythmia initiation mechanisms, or is there an underlying common mechanism?
In this study, human whole heart computational simulations were used to investigate the mechanism of arrhythmia initiation in LQT1, LQT2, and LQT3. Our results reveal a common mechanism of arrhythmia initiation that reproduces the different clinical pause-independent and pause-dependent characteristics for LQT1 vs LQT2 and LQT3. In all three subtypes however, elevating ICaL in the setting of QT prolongation results in premature ventricular complexes (PVCs) arising spontaneously from the repolarization gradient, which then propagates unidirectionally and can directly degenerate into sustained arrhythmias without the need for a conduction block substrate. Since the PVCs arise from the repolarization gradient itself, we call this phenomenon “R-from-T”, differing from the traditional “R-on-T”. Because of this common mechanism, we are able to design a single universal therapy for all subtypes of LQTS, in which a 5mV shift in ICaL steady state inactivation was able to abolish the arrhythmias, regardless of the specific underlying ionic causes of LQTS.
-
College
AMC
-
Zoom
https://ucla.zoom.us/j/91001807814?pwd=L0ZxUG5NUDdkV0FUVmVHanlpUXFwdz09
-
PDF