-
Author
Maxwell Wang -
Co-author
Simi K. Singh, MD
-
Title
Rapidly extensive recurrence of esophageal neuroendocrine carcinoma following complete pathologic response to definitive chemoradiation
-
Abstract
INTRODUCTION:
Esophageal neuroendocrine carcinoma (eNEC) is a rare (<3% of US esophageal cancers) and aggressive malignancy (<5% 5-year overall survival [OS]) that lacks treatment guidelines. The timing and nature of relapse after successful treatment is not well characterized. We report a case of rapidly extensive recurrence after complete pathologic response (cPR) to definitive chemoradiation (dCRT) and maintenance therapy (MT).
CASE DESCRIPTION:
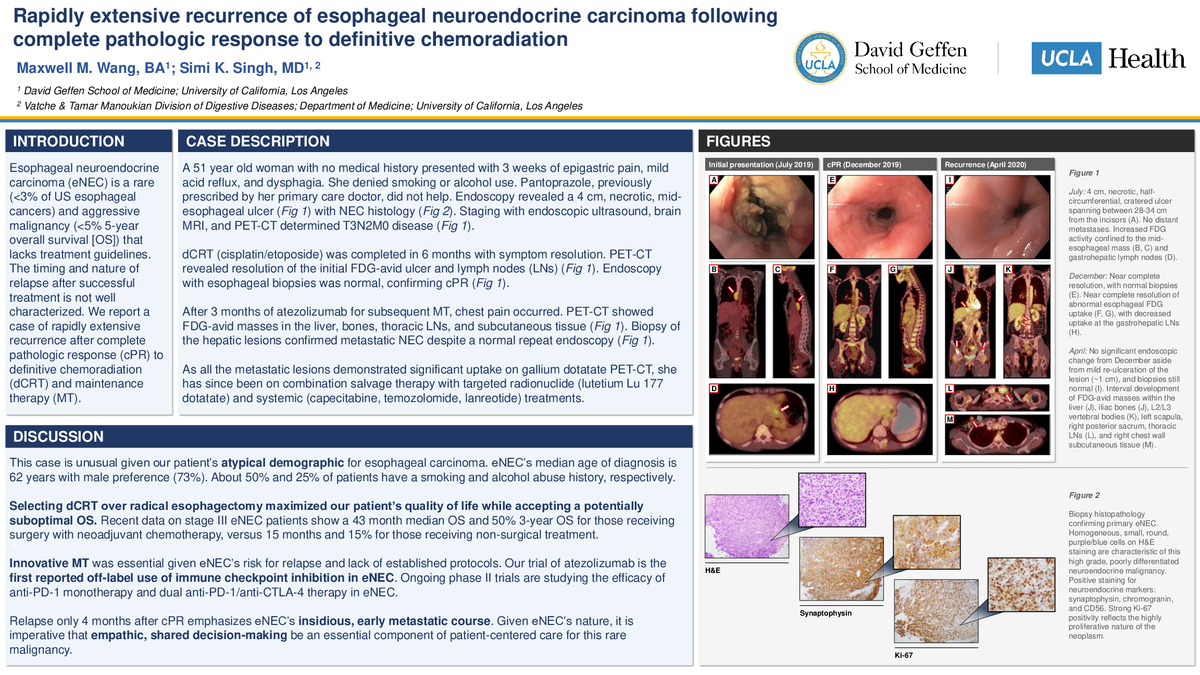
A 51 year old woman with no medical history presented with 3 weeks of epigastric pain, mild acid reflux, and dysphagia. She denied smoking or alcohol use. Pantoprazole, previously prescribed by her primary care doctor, did not help. Endoscopy revealed a 4 cm, necrotic, mid-esophageal ulcer with NEC histology. Staging with endoscopic ultrasound, brain MRI, and PET-CT determined T3N2M0 disease.
dCRT (cisplatin/etoposide) was completed in 6 months with symptom resolution. PET-CT revealed resolution of the initial FDG-avid ulcer and lymph nodes (LNs). Endoscopy with esophageal biopsies was normal, confirming cPR.
After 3 months of atezolizumab for subsequent MT, chest pain occurred. PET-CT showed FDG-avid masses in the liver, bones, thoracic LNs, and subcutaneous tissue. Biopsy of the hepatic lesions confirmed metastatic NEC despite a normal repeat endoscopy.
DISCUSSION:
This case is unusual given our patient’s atypical demographic for esophageal carcinoma. eNEC’s median age of diagnosis is 62 years with male preference (73%). About 50% and 25% of patients have a smoking and alcohol abuse history, respectively.
Selecting dCRT over radical esophagectomy maximized our patient’s quality of life while accepting a potentially suboptimal OS. Recent data on stage III eNEC patients show a 43 month median OS and 50% 3-year OS for those receiving surgery with neoadjuvant chemotherapy, versus 15 months and 15% for those receiving non-surgical treatment.
Innovative MT was essential given eNEC’s risk for relapse and lack of established protocols. Our trial of atezolizumab is the first reported off-label use of immune checkpoint inhibition in eNEC. Ongoing phase II trials are studying the efficacy of anti-PD-1 monotherapy and dual anti-PD-1/anti-CTLA-4 therapy in eNEC.
Relapse only 4 months after cPR emphasizes eNEC’s insidious and early metastatic course. Given eNEC’s nature, it is imperative that empathic, shared decision-making be an essential component of patient-centered care for this rare malignancy.
-
College
AAC
-
Zoom
-
PDF